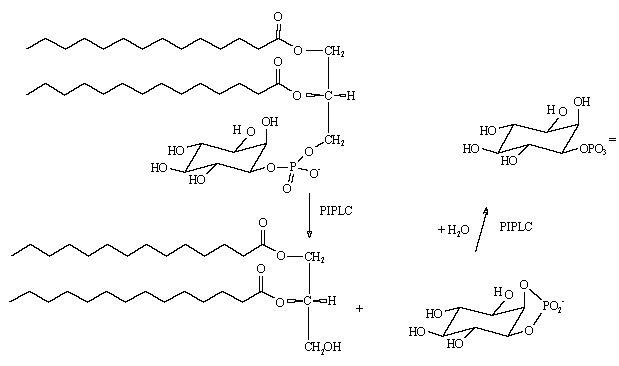
Phosphoinositide-specific phospholipase C (PI-PLC) catalyzes the hydrolysis of the phosphate diester at the sn-3 position of phosphoinositides to give a diglyceride and an inositol- phosphate ester. In mammalian cells this enzyme is involved in transmembrane signal transduction resulting in calcium mobilization and protein kinase C activation within the cell. Protozoa such as Trypanosoma brucei contain a PI-PLC which is responsible for cleavage of glycosyl phosphatidylinositol (GPI) which anchors proteins, such as the variant surface glycoprotein, to its outer membrane. Some bacteria secrete a soluble PI-PLC into the culture medium. These bacterial enzymes are able to release GPI-anchored proteins and hydrolyze phosphatidylinositol. We have chosen to study the bacterial enzyme since it is smaller (298 amino acids, 34,466 kDa), is not glycosylated, and is more easily expressed in a bacterial host as compared to the eucaryotic enzyme. It should serve as a paradigm for the mammalian enzyme.
Sphingolipids constitute a chemically and functionally diverse class of biomolecules structurally based on sphingosine. They were first described by Thudichum in 1884 and named after the mythical Sphinx, "one whose character seems mysterious." The wealth of information which has accumulated on the role of membrane phospholipids in signal transduction is mostly restricted to glycerophospholipids; only recently has the mysterious role of sphingolipids begun to be revealed. The role of sphingosine as an inhibitor of protein kinase C led Hannun and Bell in 1989 to postulate a role for sphingolipids in signal transduction. Sphingomyelinase is a phospholipase C-like enzyme specific for sphingomyelin; it catalyzes the hydrolysis of sphingomyelin to give ceramide and choline phosphate. Bacillus cereus produces sphingomyelinase that is part of the "enterotoxin complex" in addition to a phosphatidylcholine- specific phospholipase C and PI-PLC. The gene for B. cereus sphingomyelinase has been cloned. Very little is known about the basic enzymology of sphingomyelinases, such as binding and activation at lipid interfaces and their mechanism of action. We plan to construct an expression vector for the bacterial enzyme and begin enzyme studies similar to those were are carrying out with PI-PLC.
Phospholipases are very attractive proteins with which to study protein-lipid interactions since they act preferentially at the lipid-water interface and have a functional activity which can readily be followed. Much enzymology has been done with soluble enzymes and substrates; however, many enzymes act at membrane interfaces. The significance of this research lies in understanding the interactions of enzymes with lipid substrates at an interface. What are the recognition processes and mechanisms for enzyme binding at an interface, what activation processes take place at the interface, how is enzyme activity regulated, and what is the mechanism of catalysis? We are concerned with studying phospholipases in well-defined model systems with pure synthetic, chiral lipid substrates. This requires new approaches in the synthesis of specific lipids, the formulation of kinetic models, and techniques using model lipid membranes.
Our research focuses on interactions of PI-PLC and sphingomyelinase with various lipid membrane systems. The kinetics of these enzymes are studied using thiophosphate substrate analogs in continuous spectrophotometric assay systems with mixed-micelles or vesicles of substrate and other phospholipids or detergents. Kinetics are also studied with fluorescent-labeled lipids in a HPLC-based assay. Protein-lipid interactions are studied by fluorescence spectroscopy using intrinsic tryptophan or extrinsic substrate-analog probes, gel filtration, and centrifugation techniques. In collaboration with Professor Anne Walter, the micelle and lipid bilayer vesicle systems used in these studies are produced under carefully controlled conditions and characterized in order to understand their specific structures and any changes that may occur as a result of phospholipase activity.
A major aspect of our research on phospholipases has been the synthesis of phospholipid analogs as substrates for the study of these enzymes. We have synthesized two types of analogs: (1) thio analogs with a sulfur atom replacing an oxygen atom, which facilitate a continuous spectrophotometric assay of the enzymes for kinetic studies; and (2) fluorescent analogs, which facilitate very sensitive assays, since fluorescence can be detected at extremely low concentrations. Interest in these phospholipids by other researchers has led to a collaboration with a small specialty chemical company, Molecular Probes, in Eugene, Oregon. Over a dozen phospholipid analogs, synthesized in our laboratory, are now marketed through this company.
This research is supported by a grant (MCB9619859) from the National Science Foundation.